Research progress on corrosion behavior and protection technology of typical cu-ni alloy in marine environment
Release time:2022-02-28Click:964
In the Marine Environment, the complex corrosion environment such as high humidity and salinity leads to the corrosion of marine service materials more easily, and the corrosion degree is often more serious than other environments. The materials exposed to this environment are mainly corroded by seawater, microorganisms, atmosphere and carbon dioxide. Among them, seawater corrosion is mainly caused by various electrochemical reactions between metals and ions in seawater, and microbial corrosion is mainly caused by the adhesion of bacteria and other marine organisms in seawater to the surface of materials, atmospheric Corrosion [3] is a serious corrosion phenomenon caused by high humid heat and high chloride ion concentration in a complex environment The corrosion of carbon dioxide [4] is caused by the change of Ph and the formation of carbonic acid in seawater. The main corrosion types in marine environment can be divided into pitting corrosion, crevice corrosion, stress corrosion, galvanic corrosion and intergranular corrosion. The corrosion mechanism of Cu-ni Alloy in marine environment is often the result of synergistic action of many factors, so the corrosion type of cu-ni alloy may be a combination of many types. In order to achieve the goal of effective corrosion protection, it is necessary to take different measures according to different corrosion mechanisms to control the occurrence and development of corrosion.
With the increasing attention to the development of marine strategic resources in China, the requirements for the corrosion resistance of marine vessels are becoming higher and higher. Cu-ni alloy is playing an increasingly important role in corrosion resistance of Ocean. This is because the cu-ni alloy has good plasticity, weldability and toughness, and most importantly, on the basis of its good corrosion resistance, it also has the characteristics of resistance to marine fouling, cU-NI alloy is widely used in marine vessels, offshore oil platforms and offshore facilities. The copper-nickel alloy has many kinds and wide application range, at present, B10(C70600) and B30(C71500) alloys are the most widely used cu-ni alloys in marine environment. The two cu-ni alloys are mainly discussed in this paper. The basic chemical compositions of the two commonly used cu-ni alloys are shown in Table 1.
Copper-nickel alloy has been widely used in marine engineering because of its resistance to fouling by marine microorganisms and its excellent resistance to adhesion and corrosion of marine organisms. The corrosion of cu-ni alloy on the surface of cu-ni alloy is different because of the different antibacterial ability of cu-ni alloy to different strains. San Et Al. [12] studied the corrosion failure of cu-ni alloy coatings by Aeromonas and DA bacteria (which are common in marine water pipes) . The results show that the adhesion of these two bacteria can cause uneven surface of cu-ni alloy, moreover, the bacterial adhesion layer on the surface of cu-ni alloy and the metabolites and possibly acidic groups in EPS can increase the corrosion of cu-ni alloy and cause crevice corrosion by decreasing the interface Ph. CU-NI alloys are more susceptible to SRB bacteria and more prone to corrosion than Aeromonas and DA bacteria. CU-NI alloys show excellent corrosion resistance in seawater without SRB bacteria.The results show that the antibacterial property of Cu-ni alloy is due to the formation of a Cu-enriched film on the surface of the alloy, and the copper salt and Cuprous oxide in the film play an important role in the antibacterial property. Therefore, some researchers for the antibacterial copper-nickel Alloy summary, for the antibacterial copper-nickel alloy proposed in the Marine Environment Scaling Factor K. In some cases, K = a + a/t, where a is the system constant determined during the first year of exposure and t is the time in years, which plays a considerable role in the apparent antifouling properties of high copper alloys. The Scaling Parameter K is defined to allow quantitative treatment of the effect of scaling on the surface corrosion rate for the first time. Where K & GT; 1, Scaling Will Aggravate Corrosion, and K & Lt; 1 will inhibit corrosion [15].
1 Corrosion Mechanism of Cu-ni Alloy in marine environment. In Marine Environment, a passivation film or corrosion product film is usually formed on Cu-ni alloy. It has been reported that the electrochemical stability and corrosion resistance of the passivation film are significantly affected by the composition change of the passivation film. There are three types of point defects in passive films: Cation Vacancy, anion vacancy and cation gap, which correspond to electron acceptor, electron donor and electron donor respectively. In this case, the Anion Radii of Ni and Cu in the periodic table are similar (Cu + is 0.77 ? and NI2 + is 0.69 ? [20]) because of their close proximity to each other, which means that Cu + or vacancies favour the substitution of NI2 + and do not favour the formation of interstitial ions. Previous studies have shown that in order to have the lowest formation energy, Cu + is more easily formed in the passivation film than in the copper gap ion. Therefore, the anion vacancy is more advantageous in the passivation film of Cu-ni Alloy, oxygen vacancies, which tend to form more easily than the other two defects. Because of the passivation film on the surface of cu-ni alloy, cu-ni alloy has better corrosion resistance.
In order to study the corrosion resistance of cu-ni alloy, it is necessary to understand the corrosion mechanism of cu-ni alloy. The CU-NI alloy was most affected by Cl-in seawater. It is found that the addition of Cl-to seawater increases slightly the charge transfer resistance of B30 alloy, which means that B30 alloy has good corrosion resistance in seawater containing Cl-. In seawater, copper in cu-ni alloy reacts more with Cl-than with Oh-, so Cl-is the main corrosion factor in seawater. CL-IN seawater will lead to corrosion of Cu-Ni alloy, and Cu and Ni will change correspondingly during the corrosion.
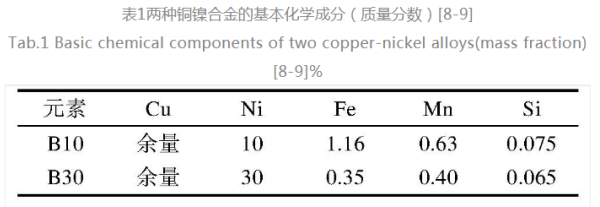
During the early erosion process of Cu-ni alloy, Cu was mainly transformed into cucl2-[23-24] , and the main process was as shown in equation (1) FO (3) . In the middle period of corrosion, if CuCl2-is enriched near the surface of cu-ni alloy, it will lead to hydrolysis reaction and formation of Cu2O. At the later stage of corrosion, if CU2O is subjected to further chemical transformation in seawater at 25 °C, it may be oxidized on the surface of the alloy and turn into corrosion product film, which mainly contains Cuo or Cu2(OH)3Cl, this film will also protect the CU-NI alloy. The reaction equation depends on the Ph of the environment, when the reaction takes place in alkaline and neutral environments (5) , in acidic environments (6) .
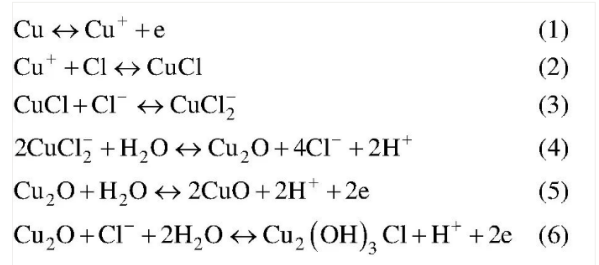
Ni in cu-Ni alloy can be transformed into NICL2[27] through two processes during the early erosion process. The main process is as shown in formula (7) as (8) . In the later period of corrosion, because the Ph of seawater is generally alkaline, and the Ph will gradually become neutral with the increase of seawater depth, so in the later period of corrosion it will be further converted into Ni (OH)2. The main process is shown in equation (9)(10) .
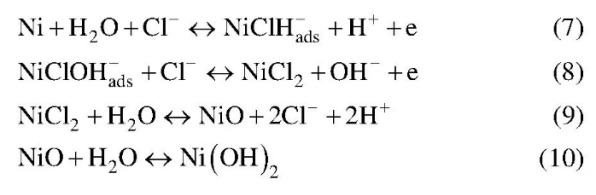
The semi-passivated Oxide Film on the surface of cu-ni alloy can protect the alloy from corrosion, but the oxide film will fall off under the action of higher concentration Cl-. Thus the reaction of formula (11) will occur.
2 study on corrosion behavior of cu-ni alloys in marine environment at present all CU-NI alloys on the market have good corrosion resistance, but for B10[29] and B30 cu-ni alloys, which are most widely used, b30 Alloy has better corrosion resistance than B10 alloy. The corrosion resistance of B10 alloy is better than that of B30 alloy when seawater is turbulent. Therefore, the two alloys show different corrosion resistance under different marine environment, and their corrosion behaviors are also different.
2.1 corrosion behavior of cu-ni alloy in different areas in the marine environment the corrosion resistance of cu-ni alloy is affected by the velocity of seawater and the service position of cu-ni alloy in seawater. The service locations of the alloys in Marine Environment can be divided into atmospheric region, splash region, tidal region, immersion region and sediment region. In Marine Environment, the corrosion conditions are different in each corrosion region, but in general, splash region is the most serious, followed by tidal region and atmospheric region, and total immersion region and sediment region are the least. In the splash zone of the ocean, the splash zone of the ocean was simulated by the researchers because the corrosion of the cu-ni alloy was more likely to occur in the splash zone of the ocean, the corrosion resistance of cu-ni alloy was studied. Wang Hongren et Al. [32] studied the film formation process of B10 Cu-ni alloy and analyzed the composition information of the film layer by using a rotating drum erosion corrosion testing machine to simulate the scour process of splash zone in seawater, the effects of flow rate and corrosion time on the film formation process were discussed. The results show that the B10 alloy can form a double layer of dense Cu2O corrosion product film on the inner and outer surfaces of the alloy in static and flowing seawater, the corrosion product film will be washed and thinned by the action of fluid mechanics until it falls off.
The corrosion environment in the whole immersion zone is also very bad. The static water pressure and the action of marine organisms can cause serious corrosion of the alloy. The corrosion of cu-ni alloy in 3.5% NACL solution was studied by Hu et Al. . The influence of hydrostatic pressure on the corrosion behavior of B10 cu-ni alloy was studied, the chemical reaction process of cu-ni alloy before, during and after corrosion was explained in detail. The results show that the high hydrostatic pressure can enhance the activity of Cl-by promoting its adsorption on the surface of cu-ni alloy and accelerating the dissolution of the alloy. In the later corrosion cycle, the alternating hydrostatic pressure accelerates the detachment of corrosion products and the formation of macro-cracks, and the high hydrostatic pressure may promote the Cl-permeation into the CU2O lattice to produce more lattice defects, this leads to a decrease in the protection of corrosion products. Therefore, the corrosion resistance of cu-ni alloy decreases with the increase of service depth in seawater immersion zone. The alloy will also be seriously corroded in the tidal zone, and the corrosion will be accelerated in the long cycle dry-wet alternate environment. Therefore, when Et2dtc complex solution is stagnant and flowing in the simulated ocean environment, it will form the product film on the surface of cu-ni alloy. This nickel-based corrosion product film has anti-corrosion effect on the material itself, with the increase of NI content, the protective efficiency of the product film is higher.
2.2 study on corrosion behavior of cu-ni alloys in seawater environment most of researchers’marine environmental tests are carried out in laboratory, but the study on Cu-ni alloys in seawater environment is relatively few, it is proved that the cu-ni alloy has good corrosion resistance under both simulated and real sea exposure conditions. The corrosion behavior of B10 cu-ni alloy in simulated seawater was studied by Liu Tianjiao et Al [35] . The variation of corrosion rate of B10 cu-ni alloy and the growth and failure process of oxide film were analyzed. The results show that the formation and destruction of the oxide film on the surface of cu-ni alloy reduces the transient corrosion rate at first and then increases it. The corrosion product contains Cu2(OH)3Cl and Cu2O, and the corrosion product film has certain corrosion resistance, which is consistent with the above corrosion mechanism. In order to study the surface passivation film of cu-ni alloy in real sea environment. Ma Et Al. [36] studied the composition and structure of B10 cu-ni alloy films formed on the surface of B10 cu-ni alloy after immersion in the sea for one month. The results showed that Fe and Ni were enriched in the corrosion product film on the inner and outer surfaces of the alloy after immersion for one month. The corrosion resistance of the alloy with this film was found to be better than that of the untreated alloy by EIS. It is also explained in detail that the most Fe enriched in the outer layer of the alloy is in the form of γ-feooh and Fe, and the most Ni enriched in the inner layer is in the form of Nio/ni (Oh)2 and Metal Ni. It is consistent with the corrosion mechanism mentioned above.
2.2.1 study on corrosion behavior of cu-ni alloy in different seawater environment. It was found that corrosion product film was formed on the surface of cu-ni alloy after immersion in seawater. These films can protect the alloy in the real sea environment. The Sea Environment is very complex. Not only Cl-has an effect on cu-ni alloy, but other factors also lead to corrosion of the alloy. It is also necessary to study the effects of temperature, current velocity and stress on Cu-ni Alloy in marine environment. Ezuber et Al. [37] studied the corrosion effects of B10 cu-ni alloy on environmental parameters such as temperature, carbon dioxide and oxygen. The corrosion behavior of B10 alloy in aerated 3.5% NACL solution at 25 °C, 50 °C and 80 °C with and without carbon dioxide was investigated. The results show that the corrosion rate of cu-ni alloy increases with the increase of temperature and decreases with the existence of CO2, but the existence of CO2 makes the alloy more prone to surface pitting.
In addition to the corrosion of cu-ni alloy in seawater, the marine ecological environment also has an effect on the corrosion of cu-ni alloy. The corrosion behavior of B10 and B30 cu-ni alloys in different marine environments has been studied by Yang Bojun et Al. [38] . It is found that the corrosion of Cu-ni Alloy in fresh sea water is more serious than that in sea water, and the corrosion depth is nearly twice, about 0.005 MM/A and 0.011 MM/A. The corrosion rate of the alloy increases with the increase of seawater velocity in natural environment. The corrosion behavior of B30 cu-ni alloy was also investigated by Fan Xuwen et Al. . [39] . The results show that the corrosion rate of B30 CU-NI alloy increases first and then decreases with the increase of seawater flow rate. The existence of stress and strain in cu-ni alloy also has an effect on corrosion. Drach et Al. [40] studied two kinds of cu-ni alloys and monitored the corrosion of the alloys in the Atlantic Ocean for one year. The results show that the corrosion rate of cu-ni alloy is maintained at 0.02 MM/A. The corrosion rate was 39% higher than that of the untensioned fixed configuration after 12 months of exposure under tensile load. Therefore, it is necessary to avoid excessive internal stress of cu-ni alloy in marine environment in order to slow down the corrosion of materials.
2.2.2 corrosion behavior of cu-ni alloy in seawater pipeline cu-ni alloy is also subject to severe corrosion in seawater pipeline. Pipelines in the marine environment are often washed by sea water, and some pipelines are also affected by temperature, adhesion of marine organisms, and corrosion by some ions. Therefore, some researchers have studied the corrosion behavior of B30 alloy service seawater pipeline system in seawater environment where sulfides exist, and found that sulfides can greatly increase the corrosion of cu-ni alloy pipeline. It is very important to study the corrosion of welding seam and coupling area of marine water pipeline, which directly affects the safety of ship. Therefore, Zhu Weiming et Al. [42] studied the annular corrosion of B10 alloy in the vicinity of the weld, the terrace corrosion in the position of the short pipe joint and the pitting corrosion near the heat affected zone of the weld inside the elbow, after measuring its potential and current, the law of electrolytic corrosion and galvanic corrosion are summarized. The results show that the corrosion rate of B10 Cu-ni alloy is lower than that of B10 cu-ni alloy when B10 alloy and H62 brass are directly coupled in seawater, and the corrosion rate remains in a small range, about 0.03 MM/A. Brass will be seriously corroded by galvanic corrosion, the corrosion rate increases greatly with the exposed area, and the larger the area ratio of cathode and anode, the more significant galvanic corrosion effect. It is shown that B10 alloy has good protection when it is coupled with brass due to the effect of galvanic corrosion, but it damages other parts directly and is unfavorable to the welding between pipes. Therefore, galvanic corrosion should also be avoided.
Similar studies have been done abroad. Din Et al [43] studied the corrosion behavior of sea water pipe in MSF distiller in marine environment. The main material of seawater pipe is B30 copper-nickel alloy. It is found that the content of CU2 + in the pipeline is increased obviously, which indicates that the copper-nickel Alloy has been seriously corroded, and the vapor side corrosion (VSC) of the copper-nickel alloy has been detected. The experimental results show that VSC corrosion can only occur when air and carbon dioxide exist together, and the corrosion loss of copper-nickel alloy pipe can be greatly accelerated by bombarding it with superlayer condensate drops, and the dissolution of CU2 + after corrosion will further catalyze the dissolution of cu-ni alloy. In the late material replacement should be replaced when the same material to avoid galvanic corrosion. 3 protection technology of Cu-ni Alloy in Marine Environment Research Cu-ni Alloy in marine environment has good corrosion resistance, but this kind of corrosion resistance is relative, materials can also undergo severe corrosion. Therefore, based on the corrosion mechanism of cu-ni alloy in the marine environment, it is necessary to carry out targeted protection technology research. At present, the protection technology of Cu-ni Alloy in marine environment is mainly studied from material itself, surface treatment, Cathodic protection and corrosion inhibitor.
3.1 The nano-structure coating of cu-ni alloy was prepared from copper sulfate solution by electrodeposition, and the size of the coating was 160nm. As the grain is refined, a dense oxide layer is obtained. However, the Cu content in the coating will greatly affect the corrosion resistance of the alloy, therefore, adding 3.87% Cu + doping to the nano-coating can improve the corrosion resistance of the coating. Therefore, the corrosion resistance of cu-ni alloy can be greatly improved by refining grain size and adding some materials. Thurber et AL [45] B30 CU-NI alloy film was prepared by Electrodeposition, and then the composite coating was obtained by infiltration of Montmorillonite into the film. The results show that the polarization resistance of cu-ni alloy infiltrated by Montmorillonite is 65% higher than that of pure cu-ni alloy, and the corrosion resistance of cu-ni alloy is improved greatly. The most basic and simplest way to improve the corrosion resistance of copper nickel alloys is to prepare copper nickel alloys by adding micronutrient. The results of Taher et al show that the addition of Fe, AL, CR, co, Ti and micronutrient to B10 alloy will obtain better mechanical properties, and the addition of Al can effectively enhance the corrosion resistance of B10 alloy. Therefore, reasonable addition of micronutrient can effectively improve the corrosion resistance and other properties of cu-ni alloy.
3.2 surface treatment other researchers have obtained better corrosion resistance by surface treatment of cu-ni alloys or by changing the processing technology. The results show that the surface grains of B10 cu-ni alloy can be refined into nano-grains and the number of grain boundaries can be increased by surface rolling, the corrosion resistance of Cuo, Cu2O and Cu (OH)2 passivated films is better than that of untreated Cu-ni alloy. The cu-ni Alloy B30 was treated by friction stir treatment (FSP) and laser deposition (DMD) . Compared with DMD, it was found that FSP reduced porosity, refined grain, increased hardness, decreased ductility and increased corrosion rate, about 0.012 MM/A. The corrosion resistance of DMD is better, about 0.009 MM/A. Therefore, the material in improving corrosion resistance, but also at the expense of some other properties.
3.3 cathodic protection and corrosion inhibitor in addition, some researchers have used chemical methods to enhance the corrosion resistance of cu-ni alloy. The electrochemical protection of Fe on B10 cu-ni alloy and B30 cu-ni alloy has been studied. Under simulated marine environment, the self-corrosion potential of FE is lower than that of the two cu-ni alloys, therefore, the corrosion resistance of cu-ni alloy can be greatly improved by the CATHODIC protection method of electrochemical sacrificial anode. The galvanic corrosion of Fe and cu-ni alloy was also studied. The corrosion of cu-ni alloy was greatly inhibited by using Fe as anode material. Although the CATHODIC protection method of this kind of sacrificial anode can greatly restrain the corrosion of cu-ni alloy, it will cause loss to the anode material, and it needs to be supplemented for a period of time. Later, it was found that corrosion inhibitors can be used to slow down corrosion, corrosion inhibitors are adsorbed on the metal, to inhibit the CATHODIC and anodic reactions, and thus achieve corrosion inhibition effect [50] . Khadom et Al. [51] studied the corrosion inhibition of cu-ni alloy by Benzotriazole in simulated acidic environment. electrochemical detection showed that the corrosion inhibition efficiency was 99.8% at the concentration of 0.1 mol/l BTA and 35 °C, the corrosion current density is reduced by nearly 800% . Therefore, the corrosion inhibitor can greatly improve the corrosion resistance of cu-ni alloy.
Conclusion cu-ni alloy has been widely used in the ocean due to its unique antibacterial property. Researchers from all over the world have made great contributions to the study of the corrosion mechanism of cu-ni alloy and the improvement of its corrosion resistance. At present, the research of Cu-ni Alloy in China is far less than that in other countries, and the research gap in corrosion resistance and other physical properties is large. In the future, it is expected to carry out further research on the surface treatment of cu-ni alloy. Starting with the preparation process of cu-ni alloy, after reasonable surface treatment, corrosion inhibitor can be used to further improve the corrosion resistance.
Source: China Corrosion & Protection Network
Disclaimer: Some pictures and texts on this site are collected from the Internet and are only for learning and communication. The copyright belongs to the original author and does not represent the views of our site. This site will not bear any legal responsibility. If your rights are violated, please contact us to delete it in time.


