An interpretation of copper refining method
Release time:2022-04-21Click:889
In order to improve the use and processing performance of copper, it is necessary to remove impurities in crude copper, such as nickel, lead and arsenic, and recover valuable metals, such as gold and silver, which is the refining process of crude copper. The process is generally divided into two stages, one is to refine crude copper into anode copper by pyrometallurgy, and the other is to electrorefine anode copper into electrolytic copper.
In order to improve the use and processing performance of copper, it is necessary to remove impurities in crude copper, such as nickel, lead and arsenic, and recover valuable metals, such as gold and silver, which is the refining process of crude copper. The process is generally divided into two stages: one is to refine crude copper into anode copper by fire method, and the other is to electrolytic refine anode copper into electrolytic copper.
1. Pyrorefining
Pyrorefining is the process of crude copper anode copper. The basic process is to blow air into the crude copper melt in the refining furnace to oxidize the impurities with high oxygen affinity in the melt, such as zinc, iron, lead and nickel, float on the melt surface in the form of oxide to form slag, or volatilize into the furnace gas and remove it. After the residual oxygen is removed by reduction, it can be cast into anode plate.
To sum up, this process can be divided into five steps: feeding, melting, oxidation, reduction and pouring. In the reduction step, at present, the mainstream reducing agents include ammonia, liquefied petroleum gas and heavy oil. In China, heavy oil is the main reducing agent, and about 4kg reducing agent is needed per ton of copper.
Refining furnaces generally include fixed refining reverberatory furnace, rotary refining furnace and tilting refining furnace. Among them, the rotary refining furnace has good refining effect, high product quality, energy saving and low production cost, and has good economic benefits.
In addition to the anode plate (containing 99.2% - 99.7% Cu), the refined products also include slag, furnace gas and smoke. Among them, the copper content of slag is high, about 10% - 30%. Generally, it needs to be returned to the converter for blowing or added to the blast furnace for further treatment. The composition of furnace gas is generally O2, CO2 and Co, without SO2, so it can be discharged directly.
Electrolytic refining is the process of anode copper refining copper. The basic process is to immerse the anode plate and starting plate (pure copper or stainless steel) into the electrolyte (aqueous solution of sulfuric acid and copper sulfate) at the same time in the electrolytic cell. Under the action of direct current, the copper on the anode enters the electrolytic solution in an ionic state and is electrochemically precipitated on the cathode to become cathode copper (electrolytic copper), and the valuable metal is precipitated and recovered in the form of anode slime. The basic principle of this method is to separate copper and impurities by using their different potential sequences.
In the traditional electrolysis process, the cathode starting plate is mainly made of pure copper. This process is relatively complex and requires an independent production system, with high labor intensity. In the production process, the starting plate needs to be replaced regularly to increase the cost.
After the 1970s, the permanent stainless steel cathode method was put into use, that is, the stainless steel starting plate was used as the cathode, and the cathode copper was stripped from the stainless steel cathode by the automatic stripping machine. This method not only does not need to establish a separate starting plate production workshop, but also can greatly reduce the cycle of replacing the starting plate, so as to reduce the production and labor cost. At present, this method has been widely used in smelters at home and abroad. Permanent stainless steel cathode method can be divided into isa method, Kidd method and ot method, with the same basic form and slightly different details.
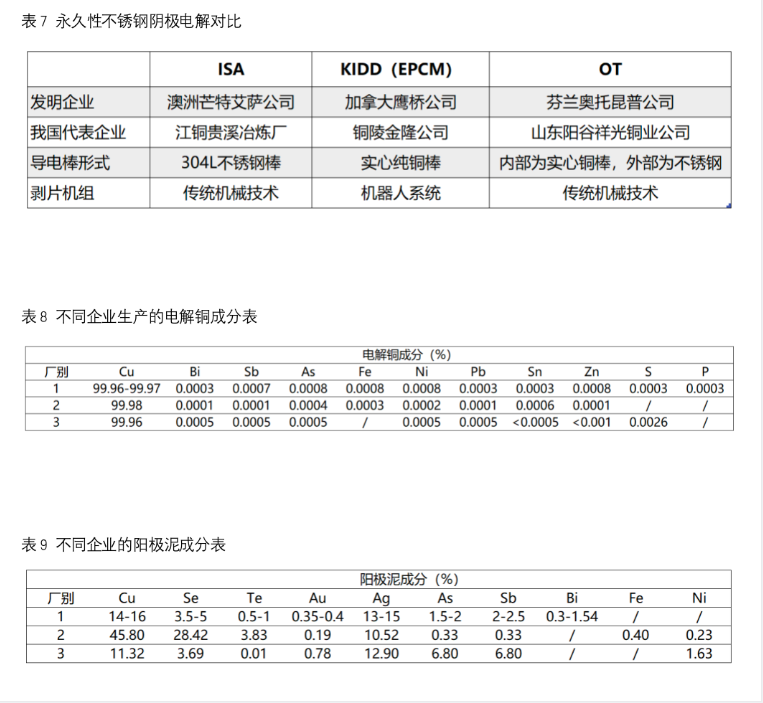
Source: Changjiang nonferrous metals network


